Le Thuy G; Nguyen Nghi H; Ruan Banfeng; Baell Jonathan B; Kundu Abhijit (TCGLS Member); Ghoshal Atanu (TCGLS Member); Preston Sarah; Jiao Yaqing; Chang Bill C H; Garcia-Bustos Jose; Jabbar Abdul; Gasser Robin B; Preston Sarah; Ruan Banfeng; Xue Lian; Huang Fei; Baell Jonathan B; Keiser Jennifer; Keiser Jennifer; Hofmann Andreas; Wells Timothy N C; Palmer Michael J
Journal of Medicinal Chemistry 2018, 61(23), 10875-10894
DOI: https://doi.org/10.1021/acs.jmedchem.8b01544
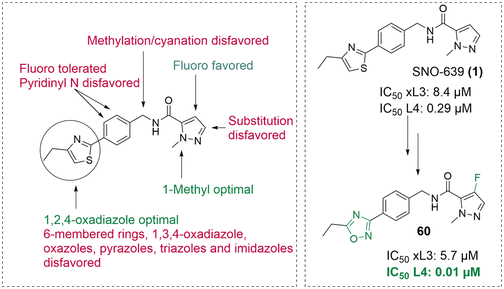
Abstract: A phenotypic screen of a diverse library of small molecules for inhibition of the development of larvae of the parasitic nematode Haemonchus contortus led to the identification of a 1-methyl-1 H-pyrazole-5-carboxamide derivative with an IC50 of 0.29 μM. Medicinal chemistry optimization targeted modifications on the left-hand side (LHS), middle section, and right-hand side (RHS) of the scaffold in order to elucidate the structure-activity relationship (SAR). Strong SAR allowed for the iterative and directed assembly of a focus set of 64 analogues, from which compound 60 was identified as the most potent compound, inhibiting the development of the fourth larval (L4) stage with an IC50 of 0.01 μM. In contrast, only 18% inhibition of the mammary epithelial cell line MCF10A viability was observed, even at concentrations as high as 50 μM