Novel 1-Methyl-1H-pyrazole-5-carboxamide Derivatives with Potent Anthelmintic Activity
Thuy G. Le, Abhijit Kundu (TCGLS Member), Atanu Ghoshal (TCGLS Member), Nghi H. Nguyen, Sarah Preston, Yaqing Jiao, Banfeng Ruan, Lian Xue, Fei Huang, Jennifer Keiser, Andreas Hofmann, Bill C. H. Chang, Jose Garcia-Bustos, Timothy N. C. Wells, Michael J. Palmer, Abdul Jabbar, Robin B. Gasser, and Jonathan B. Baell
Journal of Medicinal Chemistry 2019 62 (7), 3367-3380
DOI: 10.1021/acs.jmedchem.8b01790
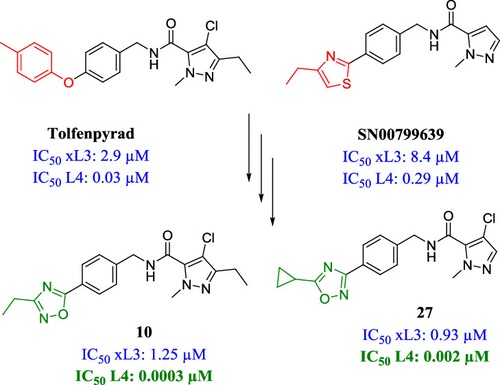
Abstract: A phenotypic screen of two different libraries of small molecules against the motility and development of the parasitic nematode Haemonchus contortus led to the identification of two 1-methyl-1H-pyrazole-5-carboxamide derivatives. Medicinal chemistry optimization-targeted modifications of the left-hand side, middle section, and right-hand side of the hybrid structure of these two hits to elucidate the structure-activity relationship (SAR). Initial SAR around these hits allowed for the iterative and directed assembly of a focused set of 30 analogues of their hybrid structure. Compounds 10, 17, 20, and 22 were identified as the most potent compounds, inhibiting the development of the fourth larval (L4) stage of H. contortus at sub-nanomolar potencies while displaying strong selectivity toward the parasite when tested in vitro against the human MCF10A cell line. In addition, compounds 9 and 27 showed promising activity against a panel of other parasitic nematodes, including hookworms and whipworms.