Published in: ChemistrySelect, Volume9, Issue30, August 12, 2024, e202400574
DOI: 10.1002/slct.202400574
Authors: Nazir Uddin, Sudipta Roy [TCGLS Member], Sayantan Roy [TCGLS Member], Dipankar Paul, Geetanjali Basumatary, Gitish K. Dutta, Paresh Nath Chatterjee
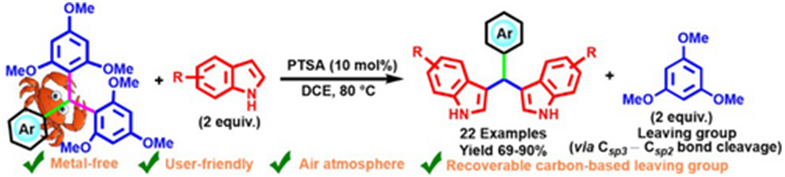
Abstract: An alternative approach to synthesize bis-indolylmethanes (BIMs) via a unique p-toluenesulfonic acid (PTSA) catalyzed dual Csp3−Csp2 bond-breaking reaction has been reported here. In the reported protocol, 1,3,5-trimethoxybenzene (TMB), an electron-rich and sterically bulky arene, acts as a carbon-based leaving group which can be recovered after the completion of the reaction. In this process, several BIMs of various indole derivatives can be synthesized from symmetrical triarylmethanes (TRAMs) containing bis-TMB motifs. By modifying the reaction conditions, we could control the sequential bond cleavage of two Csp3−Csp2 bonds in starting TRAM.
Graphical Abstract A double C−C bond-cleaving reaction under mild PTSA-catalyzed conditions to synthesize bis-indolylmethanes is reported. 1,3,5-Trimethoxybenzene, which not only acts as a good leaving group but also is recoverable and reusable. The conceptually unique method explores by-product re-utilization in chemical synthesis, making the current protocol an illustration towards sustainability.