Published in: Synthesis, January 2025
DOI: 10.1055/s-0040-1720160
Authors: Manna, Priyadarshi [TCGLS Member]; Chatterjee, Arunima [TCGLS Member]; Kundu, Mrinalkanti [TCGLS Member]; Rao, G. Prabhakar [TCGLS Member]; Mukherjee, Rusmita [TCGLS Member]; Podder, Swarnali [TCGLS Member]; Datta, Bandita; Adhikari, Susanta
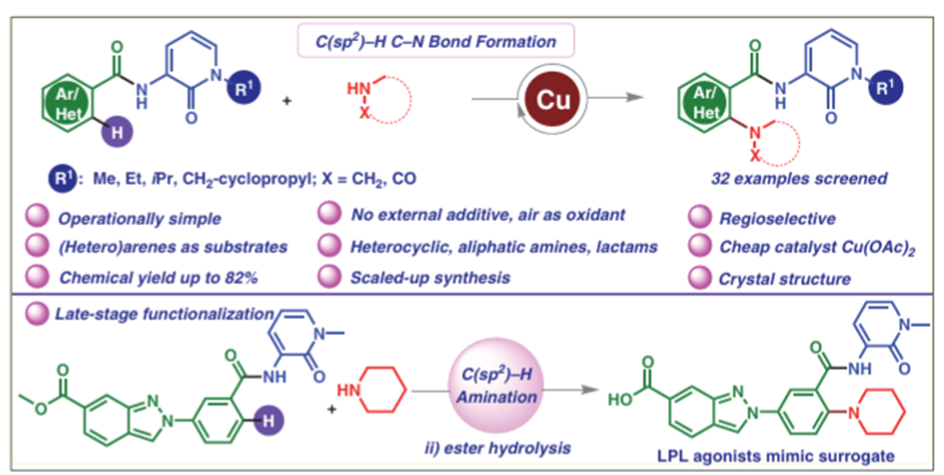
Abstract: Arylamines are essential building blocks that are found in biol. important substances, agrochems., and natural products. One of the C-N bond formation methods that is conveniently step- and atom-economical is the C-H bond activated amination process. We divulge an operationally simple and general method using 3-amino-1-methyl-1H-pyridin-2-one (AMP) as inbuilt directing group (DG) for additive-free, copper(II)-catalyzedorthoamination of β-C(sp2)-H bonds of arenes and heteroarenes. Notably, this cross dehydrogenative amination reaction exhibits a broad scope regarding amine coupling partners, including heterocyclic amines, secondary aliphatic amines, and cyclic amides, with exclusive site selectivity and excellent functional group tolerance. Moreover, implementing this methodol., we could also synthesize medicinally important compounds to showcase the suitability of this inbuilt DG for late-stage functionalization.