Pati Tanmay K (TCGLS Member); Debnath Sudipto; Maiti Dilip K; Kundu Mrinalkanti (TCGLS Member); Khamrai Uttam (TCGLS Member)
Organic Letters 2018 20(13), 4062-4066
DOI: https://doi.org/10.1021/acs.orglett.8b01618
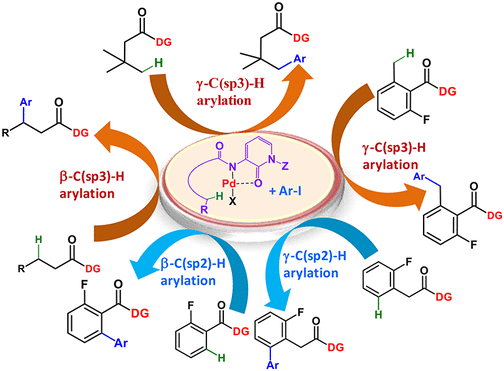
Abstract: A new bidentate directing group, 3-amino-1-methyl-1 H-pyridin-2-one, is introduced to achieve a powerful Pd(II) metallacycle for selective γ-C(sp(3))-H activation and arylation of aromatic and aliphatic carboxylic acid derivatives. The versatility of the directing group is validated for remote arylation of β-C(sp(3))-H, β-C(sp(2))-H, and γ-C(sp(2))-H to achieve therapeutically important 2-pyridone analogues and arylated acid synthons. The traceless removal of the directing group to retrieve the directing element and carboxylic acids makes this method more interesting.