Published in: Journal of Medicinal Chemistry, ASAP
DOI: 10.1021/acs.jmedchem.4c01154
Authors: Godwin AkpekoDziwornu,Donald Seanego,Stephen Fienberg,Monica Clements,Jasmin Ferreira, Venkata S. Sypu, Sauvik Samanta, Ashlyn D. Bhana, Constance M. Korkor,Larnelle F. Garnie, Nicole Teixeira, Kathryn J. Wicht, Dale Taylor, Ronald Olckers, Mathew Njoroge,Liezl Gibhard, Nicolaas Salomane, Sergio Wittlin, Rohit Mahato(TCGLS Member), Arnish Chakraborty(TCGLS Member), Nicole Sevilleno,Rachael Coyle, Marcus C. S. Lee, Luiz C. Godoy, Charisse Flerida Pasaje, Jacquin C. Niles, Janette Reader, Mariette van der Watt, Lyn-Marié Birkholtz, Judith M. Bolscher,Marloes H. C. de Bruijni, Lauren B. Coulson, Gregory S. Basarab, Sandeep R. Ghorpade,and Kelly Chibale.
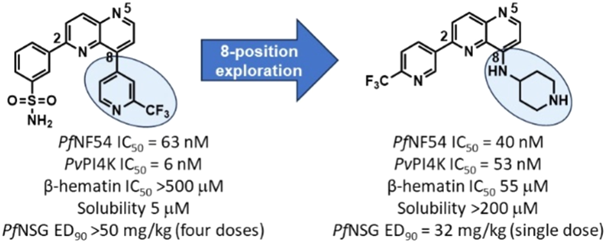
Abstract: Structure−activity relationship studies of 2,8-disubstituted-1,5-naphthyridines, previously reported as potentinhibitors of Plasmodium falciarum (Pf) phosphatidylinositol-4-kinase β (PI4K), identified 1,5-naphthyridines with basic groups at8-position, which retained Plasmodium PI4K inhibitory activity butswitched primary mode of action to the host hemoglobindegradation pathway through inhibition of hemozoin formation.These compounds showed minimal off-target inhibitory activityagainst the human phosphoinositide kinases and MINK1 andMAP4K kinases, which were associated with the teratogenicity andtesticular toxicity observed in rats for the Pf PI4K inhibitor clinicalcandidate MMV390048. A representative compound from theseriesretained activity against field isolates and lab-raised drug-resistant strains of Pf. It was efficacious in the humanized NSG mousemalaria infection model at a single oral dose of 32 mg/kg. This compound wasnonteratogenic in the zebrafish embryo model ofteratogenicity and has a low predicted human dose, indicating that this series has the potential to deliver a preclinical candidate formalaria.