Published in: Medical Oncology, January 2025
DOI: 10.1007/s12032-025-02605-8
Authors: Laavanya Das, Subhadip Das [TCGLS Member]
Abstract: Cancer is a major global health issue that is usually treated with multiple therapies, such as chemotherapy and targeted therapies like immunotherapy. Immunotherapy is a new and alternative approach to treating various types of cancer that are difficult to treat with other methods. Although immune checkpoint inhibitors have shown promise for long-term efficacy, they have limited effectiveness in common cancer types such as breast, prostate, and lung. Some patients do not respond to immunotherapy, while others develop resistance to the treatment over time, which is classified as primary or acquired resistance. Cancer immunotherapy, specifically immune checkpoint inhibitor-based resistance involves multiple factors such as genes, metabolism, inflammation, and angiogenesis. However, cutting-edge research has identified the mechanisms of immunotherapy resistance and possible solutions. Current research may improve biomarker identification and modify treatment strategies, which will lead to better clinical outcomes. This review provides a comprehensive discussion of the current mechanisms of immunotherapy resistance, related biomarker modulation, and strategies to overcome resistance.
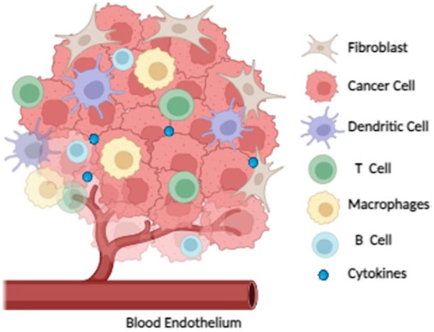
The major components of tumour microenvironment.



.jpg ?>)
.jpg ?>)
