Published in : Synthetic Communications
DOI: 10.1080/00397911.2023.2205593
Authors: Chandan K. Mahato (TCGLS Member),Subhro Mandal,Mrinalkanti Kundu (TCGLS Member)&AnimeshPramanik
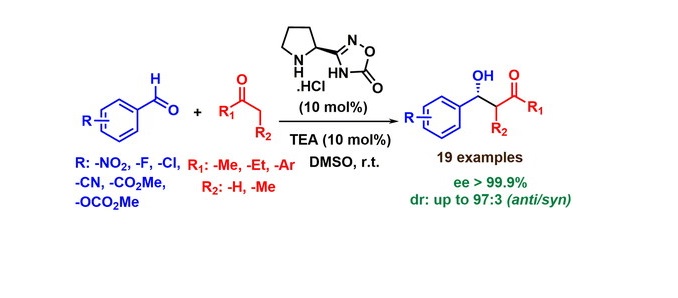
Abstract: A proline-based, chiral bi-functional organocatalyst containing both pyrrolidine and oxadiazolone heterocycle, has been successfully applied for stereoselective aldol reactions. The replacement of polar -COOH group of proline with bioisostereoxadiazolone ring provides excellent solubility of this catalyst in various organic solvents compared to low soluble proline. As a result, the organocatalyst effectively catalyzed the asymmetric condensation reaction between differently substituted aromatic aldehydes and various symmetrical and unsymmetrical ketones to produce the corresponding aldol products with excellent stereoselectivities (dr: 97:3, ee>99.9%) at room temperature in open-air.