

Dr. Benoît Laleu,Kelly Rubiano,Tomas Yeo,Irene Hallyburton,Dr. Mark Anderson,Benigno Crespo-Fernandez,Dr. Francisco-Javier Gamo,Dr. Yevgeniya Antonova-Koch,Dr. Pamela Orjuela-Sanchez,Dr. Sergio Wittlin,Dr. Gouranga P. Jana (TCGLS Member),Dr. Bikash C. Maity (TCGLS Member),Elodie Chenu,Dr. James Duffy,Dr. Peter Sjö,Dr. David Waterson,Prof. Elizabeth Winzeler,Dr. Eric Guantai,Prof. David A. Fidock,Dr. Thomas G. Hansson
Publication: ChemMedChem, e202200393
Abstract: Toolkit expansion: We report an exploration of the antimalarial compound MMV692140. The mode of resistance was identified as PfeEF2. The structural motif was explored in a chemistry program, resulting in the identification of MMV1919557, an analog with significantly improved antimalarial potency. This new series could provide a tool to further understand the potential of PfeEF2 as a target for malaria treatment
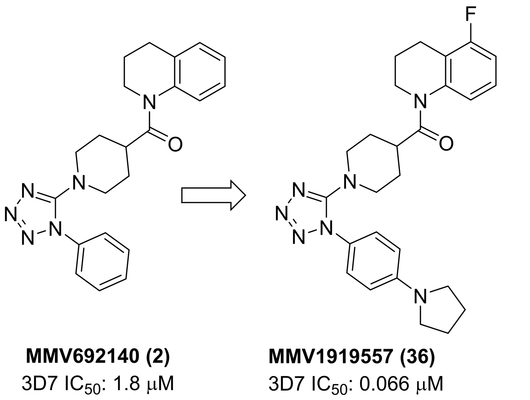
New antimalarial treatments with novel mechanism of action are needed to tackle Plasmodium falciparum infections that are resistant to first-line therapeutics. Here we report the exploration of MMV692140 (2) from the Pathogen Box, a collection of 400 compounds that was made available by Medicines for Malaria Venture (MMV) in 2015. Compound 2 was profiled in in vitro models of malaria and was found to be active against multiple life-cycle stages of Plasmodium parasites. The mode of resistance, and putatively its mode of action, was identified as Plasmodium falciparum translation elongation factor 2 (PfeEF2), which is responsible for the GTP-dependent translocation of the ribosome along mRNA. The compound maintains activity against a series of drug-resistant parasite strains. The structural motif of the tetrahydroquinoline (2) was explored in a chemistry program with its structure-activity relationships examined, resulting in the identification of an analog with 30-fold improvement of antimalarial asexual blood stage potency.