Published in : European Journal of Medicinal Chemistry Volume 280, 15 December 2024, 116921
DOI : 10.1016/j.ejmech.2024.116921
Authors : Jon Kyle Awalt, Wenyin Su, William Nguyen, Katie Loi, Kate E. Jarman, Jocelyn S. Penington, Saishyam Ramesh, Kate J. Fairhurst, Tomas Yeo, Heekuk Park, Anne-Catrin Uhlemann, Bikash ChandraMaity [TCGLS Member], Nirupam De [TCGLS Member], Partha Mukherjee [TCGLS Member], Arnish Chakraborty [TCGLS Member], Alisje Churchyard, Mufuliat T. Famodimu, MichaelJ. Delves, Jake Baum, Nimisha Mittal…Brad E. Sleebs
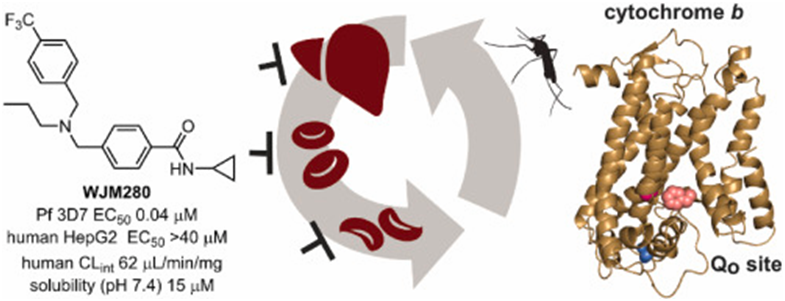
Abstract : Drug resistance against antimalarials is rendering them increasingly ineffective and so there is a need for the development of new antimalarials. To discover new antimalarial chemotypes a phenotypic screen of the Janssen Jumpstarter library against the P. falciparum asexual stage was undertaken, uncovering the cyclopropyl carboxamide structural hit class. Structure-activity analysis revealed that each structural moiety was largely resistant to change, although small changes led to the frontrunner compound, WJM280, which has potent asexual stage activity (EC50 40 nM) and no human cell cytotoxicity. Forward genetics uncovered that cyclopropyl carboxamide resistant parasites have mutations and an amplification in the cytochrome b gene. Cytochrome b was then verified as the target with profiling against cytochrome b drug-resistant parasites and a mitochondrial oxygen consumption assay. Accordingly, the cyclopropyl carboxamide class was shown to have slow-acting asexual stage activity and activity against male gametes and exoerythrocytic forms. Enhancing metabolic stability to attain efficacy in malaria mouse models remains a challenge in the future development of this antimalarial chemotype.